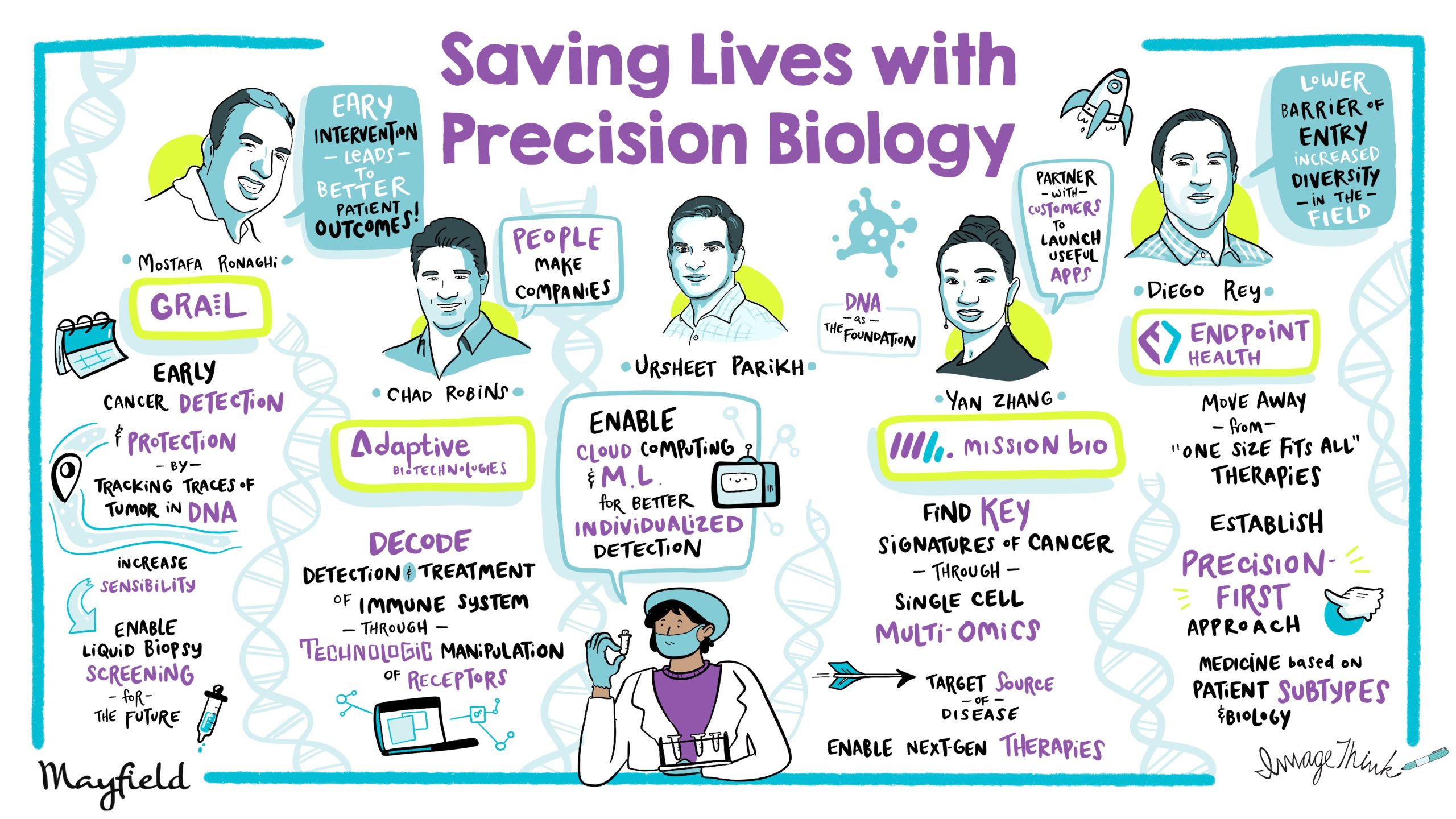
Illumina is the world leader in next-generation sequencing. Adaptive Bio is the leader in immune-driven medicine. Mission Bio pioneered single cell multi-omics for genotype and phenotype. And Endpoint is developing precision therapies for inflammatory illness, sepsis, ARDS and more. Hear from these 4 leaders who are leveraging biology breakthroughs to save lives on a wide range of topics including translating innovation from bench to bedside, aligning incentives with the ecosystem, getting to reimbursement, exploring SPACs and beyond.
Transcript
Ursheet Parikh:
The session that we had with Ugur Sahin was about BioNTech’s journey and how they became the platform company that has saved millions of lives for us this year. As you can see, BioNTech represents a new way of innovation, where advances in engineering, biology, and information technology come together to help us transform healthcare. We are now seeing the emergence of a whole new class of companies. Several of them will be created by our panelists in the room today.
It is my honor to introduce our panelists. First off, we have Chad Robins. Chad is co-founder and CEO of Adaptive Bio. They are powering the age of immune medicine. Chad has led Adaptive from concept to category leadership and a $5 billion market cap to build this enduring company.
With him, we also have Mostafa Ronaghi. Mostafa is the co-founder of GRAIL. GRAIL is one of the leading companies for early cancer detection. It was recently acquired by Illumina for $8 billion, and Mostafa has also been the CTO of Illumina, which is a leader in genomic sequencing. He’s also the CEO of SPAC.
We also have two rising stars: First, Yan Zhang, the CEO of Mission Bio. Mission Bio unlocks single cell biology to help discover, develop, and deliver new treatments for cancer cell and gene therapies.
Finally, we also have Diego Rey. Diego is a serial entrepreneur. He was also the first bio investing partner for Y Combinator, and he’s now the co-founder of Endpoint Health. Endpoint is a precision therapeutics company that is focused on immune-driven illnesses, where one of their top indications is actually critical inflammatory disorders like sepsis. That is the number one killer of people in hospitals. A lot of COVID-19 deaths have been because of sepsis, and they are soon to be starting several phase three trials.
All of our panelists have been using the engineering marriage of biology and technology, and are going to be saving so many lives. It’s truly an honor and pleasure to have all of you with me here today. Let me start with Chad. Chad, can you share with us what Adaptive does, and how that is saving lives?
Chad Robins:
Sure. Thanks Ursheet for having me on the panel today. Adaptive is an immune medicine platform. What our real goal is, is to learn how the adaptive immune system naturally sees disease so that we can diagnose disease by reading or decoding how the immune system naturally would diagnose disease, and at the same time, we can harness the power of the immune system for drug discovery.
Your immune system does two things: it detects, and it treats. So essentially, what we’ve done at Adaptive is we’ve created a series of technologies to be able to decode, down to the DNA level, your immune receptors. We can not only decode them and sequence them, we can then match them to the diseases that they see and bind to, and ultimately go in and target and kill.
We’ve developed a series of products from research, that no matter what you’re doing in the immune system, now you have a much more powerful set of tools to be able to do the research, to clinical diagnostics, to be able to diagnose disease, to drug discovery, to harness the power of the new medicine to treat disease.
We’ve got a couple of marquee partnerships that are helping us along the way. One is with Microsoft to be able to essentially create this extremely large map of how our bodies see disease. This is your T-cell to antigen map or T-cell receptor to antigen map, and that’s for early detection of disease. We also partner with Genentech in drug discovery. We’re attacking cancer in an entirely new way in cell therapy by essentially creating a personalized therapy for each patient based on what your immune system sees in your individualized cancer.
Ursheet Parikh:
Chad, that’s pretty awesome, and it’s great to see Microsoft and Genentech as strategic partners talking about the marriage of tech and bio.
Mostafa, congratulations again on the acquisition of GRAIL. It was a long journey. Well, can you tell our audience, what are the fundamental innovations in liquid biopsy that GRAIL pioneered, and how that is going to transform healthcare?
Mostafa Ronaghi:
Thank you Ursheet for having me at this panel among other friends. It is a pleasure to be here. The way we looked at blood was basically a switch in the body, and whatever happens in the body, it would end up in the blood, and you would see the traces of DNA. In the late 2000s, we started seeing a few publications showing that fetal DNA can be detected in the blood, and that grabbed our attention to look for basically traces of tumors in the blood system.
So, we started the research activities that ended up being four companies, actually, from Illumina. The first one was Guardant. The second one was AccuraGen, and then AccuraGenDX, and the last one was GRAIL.
In GRAIL, we took a fundamentally different approach. We had to increase the sensitivity by at least another hundred-fold to have the ability to detect cancer in earlier stages. We believe that cancer is a curable disease, and the best cure for cancer is actually surgery, but you have to detect it early.
So we started actually a multi-pronged approach, a technical approach, to tackle the sensitivity issue. Of course, cheaper sequencing helped a lot in that regard, and we expanded the panel, and we started looking at RNA, DNA, and mutilation, and then we decided mutilation is going to give us the sensitivity and specificity related to the tissue. So, we basically created a good tool set of technologies and technical tricks around mutilation to offer the GRAIL test, and I’m happy to see that the test was launched a couple of months ago, and we’re already actually saving lives.
Ursheet Parikh:
That’s great. So, can we expect to just go see our doctors once a year and along with our cholesterol and diabetes screening, also start getting cancer screening?
Mostafa Ronaghi:
Yes, I do believe that, actually. This kind of liquid biopsy test is going to be the standard annual checkup kind of test. In the long run, I do believe that liquid biopsy is going to be used for other diseases, and this is going to be the first line of screening tests that you would do, replacing medical imaging in the next couple of decades. It’s not going to happen in this decade, probably, as we are going to need medical imaging to compliment this data, but eventually we are going to actually replace medical imaging as a first line of screening for all kinds of diseases.
Ursheet Parikh:
Yeah, that we truly believe, and we have a company at Mayfield called Mirvie that was actually doing liquid biopsy to predict and prevent pregnancy complications, so really buy that.
Moving over to you, Yan, there’s been a war on cancer for 50 years, right? How come we’ve not cured it? How is Mission Bio helping find cures for cancer and other genetic diseases?
Yan Zhang:
Thank you so much, Ursheet, first of all, for having me at the panel amongst some of these most prestigious and brilliant colleagues here. We have been at war against cancer for 50 years. We have not been standing still. So frankly, a huge amount of technical advancement, including for example, next generation sequencing pioneered by Illumina in the past decade has built a strong foundation for us to actually much, much better understand the genomics and the underlying mechanisms and diseases, as well as the therapeutic advancement, such as what Chad had already elucidated.
I think we’re at the junction, because all of the new technologies are coming together in addition to genomics, in addition to cell biology, in addition to all of the IT and big data. We are at the cross section where we actually now have the ability and have the knowledge to go one step further, so that’s where Mission Bio comes in.
Our technology is microfluidics, where we can enable a deeper understanding of biology and of diseases and therapeutics at a single cell level. We can’t be here without all of the other advancements that have already happened in the past 50 years. We’re really lucky to be at the intersection.
So how we think about cancer is we really need to get to the root of the disease. The root of the disease is genetics. It is the protein that it produces and the pathways. It is at a single cell level. We actually, frankly, have not won the war against cancer. A big part of that is because of drug resistance. There was a small body of the cells, which we actually today, the therapeutics don’t treat, but those are the ones that continue to evolve and respond, as well as there are many other factors that really come back stronger, possibly because of a co-occurrence of many mutations and really adaptive to the therapies.
To really understand at the single cell level and understand the fundamental genetics against them, we’ll be able to help society and the community to design better drugs, design better therapies, as well as be more effective in clinical trials. So we’re really glad to play a very big role, and we believe that will potentially move treating from just prolonging life for months or years to actually curing cancer.
So that’s a big ambition, but we’re not stopping there. Using the tapestry platform at the single cell multiomics, we actually also play a very big role to be the analytical platform for cell gene therapy. As Chad mentioned, cell gene therapy is really the new frontier of treatment of not just cancer, but many genetic diseases. These rare diseases cumulatively account for hundreds of millions of people who suffer, and many of them don’t have cures, and in the past we tried to cure symptoms, but with the new gene and cell therapy, we can actually cure the disease itself by making changes to our human genome.
But it is the frontier. The frontier is going to require the whole ecosystem to help support those new therapeutic ideas to be a reality to be successful. So by deploying single cell multiomics, we can help the industry to better understand the therapeutics, to characterize and quantify the therapeutics in terms of the safety, efficacy, to actually accelerate the development and quantification, and help deliver them back to the patients. So we are extremely excited to participate and to lend our hand to this entire ecosystem so we can, as a society, win the war.
Ursheet Parikh:
Yan, that’s well summarized. I think someone I respect just mentioned that they look at you as an ETF for the whole sector, because you are powering and enabling so many… An exchange-traded fund for the whole sector, because you’re empowering and enabling so many of these next gen therapies. Diego, I got to put you on the spot. I get to work with you. Mayfield’s been partnering with amazing entrepreneurs from inception, be it founders of Genentech, Amgen, Millennium Pharmaceuticals. But when I met you for the first time, I really paused, and just to be sure I’d heard right, because you said you wanted to reinvent pharma. Endpoint is well on its way to building a new class of pharma company, so tell us how.
Diego Rey:
Sure. Thanks, Ursheet. Great to be here. Yeah, didn’t mean to really pick on any particular company, but in the past, pharma companies were built on what I would call a molecule-first approach, and it’s because this is what really worked. Taking a molecule and then turning it into a drug is a huge feat, so it made sense to start with a drug, and then to see what illnesses can the drug actually go and help. One of the issues, though, is that this approach can lead to a one size fits all therapy that doesn’t always work for everybody.
Today, the world is very different. Now we have new enabling technologies and infrastructure that we’ve been discussing here, thanks to companies like Illumina, and these things really enable us to harness a huge amount of biological data that we couldn’t before. So, instead of starting with a molecule, we can now start with a, very much like Chad said, start with individual patients and data from these individual patients, even within the same illness, and then figure out how to help each of these patients based on their unique needs.
Endpoint is a therapeutics company with what we call a precision-first approach that is really only made possible today. So, instead of starting with a molecule, what we do is we start with patient biology to develop and later commercialize new medicines based on patient subtypes that we can go in and identify. At the end of the day, we believe that this will enable enormous improvement in patient outcomes.
As an example that you highlighted earlier, in critical care, where we’re starting, this is an area where there’s more deaths than there are in all cancers combined, and at the same time, it’s an area where there are very few, if any, effective therapies. So this is an area with this approach that we think will bring to market some of the first life-saving therapies for these patients.
So, to answer your question, I think what we realized is that a fully integrated therapeutics company with a precision-first approach is not only now possible, but it’s really, really badly needed, and so we’re building it, and that’s Endpoint Health.
Ursheet Parikh:
So in many ways, what you’re saying is you’re really focusing on truly understanding the disease and subtyping the disease, and then finding what would be the best treatments for it. Well, we really wish you a lot of success because we do want to see these treatments come to market. Earlier this year I spent about 10 days in an ICU for a close family member, and that was very sobering.
Chad, moving on to more fun stuff, how are you looking at the convergence of biology and technology, and how do you see that transforming healthcare, and what is the next frontier for it?
Chad Robins:
Yeah, sure. I thought, because this is traditionally a tech conference, I would give an analogy that I think is applicable to the audience, although I do have to say that probably one of the largest opportunities in tech is this convergence and this application of technology and machine learning to biology.
But if you think about it, and because we have Mostafa here, we’ll take a little bit, think about this as 1.0, 2.0, 3.0. If you think about the hardware, 1.0 being the IBM, if you will, over time got faster, cheaper, smaller, easier to use. The same thing happened with sequencing, right? You had these large sequencers that took up pretty big areas that got smaller, faster, cheaper, easier to use, and now if you think about that, that is really 1.0. 2.0 is, what are the applications of these machines in the parlance of tech. Microsoft came along and made it so that the everyday person could use these machines. So 2.0 to me is how do you use this instrumentation for diagnosis and/or drug discovery? I think my 3.0 is really now layering on machine learning on top of that, which is not just machinery. It’s the power of cloud computing and machine learning that now can take the information that’s generated from these machines and the applications on top of these machines to then make a patient decision.
Another thing that Mostafa said is the concept of early intervention leading to better patient outcomes. So now, and I will also say that what Diego and Jason are doing at Endpoint Health is really sifting through this information combined with biological information to determine how these drugs are going to work on patients on an individualized basis based on their biology.
Specific to Adaptive, and you asked what’s next in the new frontier, I’m going to highlight our work with Microsoft, explain how that works, which is essentially… Let me first give you some biological stats. The human genome is fixed, right? You’ve got about 30,000, other than some pointless mutations, you have 30,000 genes in the human body. Your immunome, or your adaptive immune system genes, each person has about a trillion of them in the body, about a hundred million unique. In the population, there’s about 10 to the 16th or 10 to the 18th, like trillions of choices.
Why I’m saying this is because our body’s job is to be able to recognize anything, any potential invader, anything foreign that it could potentially see, find it, and then go kill it. So what we’re doing with Microsoft essentially is building this massive map between your T-cell receptors or your immune receptors that recognize disease and antigens. Antigens are signals of a disease. So, what happens is every disease has editors that are specific for that disease, and it’s a little flare, think of it. It goes up on the outside of the cell, your T-cell recognizes it, it springs into action and kills it.
So the idea in, let’s use Microsoft Excel since we were on the tech analogy, we’re essentially building this massive VLOOKUP table, disease by disease, between your T-cells and your antigens. We’re able to feed that through chemistry, some proprietary assets or technologies that we have, where we can physically start creating these connections. But that only starts it going. What happens is then Microsoft on top of that, we have all this machine learning to impute and come up with more connections that essentially build out that map.
Now the idea, and you asked about, hey, when can you go into a doctor’s office and get a screen for cancer? The idea here is that we’ll start with one disease at a time. We did this for COVID, and T-Detect. There’s a franchise called T-Detect. We did T-Detect COVID, we’re now doing it for Lyme disease. We’re also in process for irritable bowel, basically a differential diagnosis between Crohn’s, IBS, celiac. So if you walk in with the same set of symptoms, we can rule in, as opposed to a rule out test, where you go from specialist to specialist, doctor to doctor over a two-year patient odyssey, now we can definitively diagnose based on your immune receptors, based on your body, what it’s seeing, referencing this map. We were never going to get every receptor, so again, that’s where this machine learning power comes in and says, we can be 99.99% confidence that if you have a receptor, that it binds to a certain antigen, and that’s specific for the disease, so you can reverse diagnose disease from the receptor itself.
So that’s what we’re doing, and eventually going from one disease to differentiated panels or differential diagnosis to essentially an immune checkup, where you go into your doctor’s office, and we’ve mapped hundreds, if not thousands of diseases, including our approach to cancer, which is instead of looking at the cancer itself, we’re looking at the immune response to the cancer that clonally expands, and it might be complementary to some of the liquid biopsy approaches that GRAIL and others are doing. So, that’s really the next frontier for us is diagnosing disease essentially from reaping your immune system.
Ursheet Parikh:
That’s amazing. We are going to get truly a fundamental understanding of the human state and the state when things go off track, so that’s great. Mostafa, what are the big entrepreneurial and investment opportunities you see?
Mostafa Ronaghi:
I’m actually very excited about the rise of cell therapy. That’s an area that has shown a lot of recent progress, and we see that there’s actually is working on a lot of different approaches that have been implemented, so I’m very hopeful that cell therapy is going to be the next generation therapeutics, at least against immunological disorders like cancer and other diseases and so on. Chronic diseases.
The technologies that enabling actually the cell to PR, the single cell technologies and the spatial biology approaches, and I think that’s a trend that’s going to continue. We really need to have the same cost trajectory as we had for sequencing to reduce the costs, to tackle this issue, to provide much more comprehensive information to understand the cells much better. The tool sets like synthetic biology tools are very exciting now actually being implemented in cell therapy, and it’s giving you actually the specificity and the sensitivity for the therapy.
Ursheet Parikh:
Yeah. Yeah, I think that the ability to truly engineer the cells and the genome and how that creates therapeutic effect along with an ecosystem that allows for mass production on these things. I think these are so fundamental, so seminal, and clearly a big area of investment for us at Mayfield.
Diego, I wanted to get to you next. You’ve been an entrepreneur and an investor at YC. How do you see company building in engineering biology different from just a tech or a software company?
Diego Rey:
Sure, yeah. Actually, maybe to start with a similarity, based on something that Chad mentioned, he put it I think really well that I think that because of all these advancements in technology and infrastructure that we’re seeing, we’ve been discussing here on the panel, I think the barrier to entry to building companies in engineering biology has really lowered. So now, we can really build applications, just like Chad said, in an analogous way maybe that the tech industry went from, for example, building chip foundries to building apps.
So I think this has really opened the door to a lot of first-time founders, and this is now increasing diversity in our field, which is really a great thing. At the same time, I think unlike tech, engineering biology still takes a lot more time. Sometimes it could cost more, and it also takes more diligence from an investor as well, so those would be some key differences.
Another observation though is actually one from a former colleague of mine, Jared Friedman, a partner at YC, and what he pointed out was that life sciences founders, from his point of view, of all the companies YC is seeing, they now have over 400 life sciences companies. He’s noticed that life sciences founders are more hesitant to start a company, even though in many cases what they’ve developed is more mature than what most tech founders have when they’re starting their company. So, I’d say that we really need more life sciences founders to go and start their companies.
Ursheet Parikh:
Yeah. I mean, I recognize that while there’s a lot of similarities, one thing is that the business of moving atoms has always been harder than the business of electrons, right? Software has been amazing because you can sit on your laptop and just move electrons and magic happens, and so it becomes easier to iterate, pivot, all of those things like that, and it does require a different mindset and view.
But you’re right that what has been amazing is that 20 years or 10 years ago, starting one of the life sciences companies required $10 to $20 million, and now we have a very, very large number of amazing companies that we started out with the first couple of $200,000 to $300,000 with a whole ecosystem here in the Bay Area. So, that’s great.
Yan, we are clearly very impressed with you and your leadership at Mission Bio. Given all the opportunities you had, what made you pick Mission Bio as the company to lead?
Yan Zhang:
Yeah, great question. I’m still learning to be an entrepreneur, I feel like. I spent about 20 years with the larger companies, but it’s such an exciting space to be. But I think how we can be successful, is the intersection we talked about the building applications. That’s Mission Bio’s philosophy. We’re not launching just a platform, we’re really launching the applications. But in order to be successful in identifying those applications, killer apps, and actually be useful, and translate that to market acceptance, we have to collaborate and partner very closely with our customers. In this case, academic cancer center KOLs, or the pharma companies, other entrepreneurs, to make sure our tools and our applications can meet their needs.
So that’s what’s really attractive to me is that to be able to come with a commercial mindset and really collaborate with a lot of creative minds who are driven by technology, but bring those creative minds together, while really solving a practical problem with the partnership. I think that’s an amazing thing. In our small company, we have statisticians, bioinformaticians, engineers, and biologists, right? All of us, and then we come together to solve one single problem. I think that’s amazing. That’s what I think we can do together to achieve the unthinkable from the past.
Ursheet Parikh:
Got it. So it’s like you pretty much found Mission Bio to have this platform that then allowed you to build applications around different disease areas.
Yan Zhang:
That’s right.
Ursheet Parikh:
And really make it easier for various drug developers to develop that.
Yan Zhang:
Yeah.
Ursheet Parikh:
That’s great. Chad, you did a bio platform company in a decade when most bio platform companies failed, right? Is there one or two things that you can talk of as how you succeeded while most of your peers didn’t make it?
Chad Robins:
Well, truth be told, we had a vision that the immune system absolutely was a platform. Platform was a dirty word when we got started, but my brother and I had no experience in biotech, so we really had no other opportunities for funding. So we said, hey, we’re going to go with our vision, and before we raised a hundred million dollars five years later, we bootstrapped it with friends and family money.
Essentially, we’re trying to land a rocket booster on a platform in the middle of the ocean on a pretty frequent basis, and in order to do that, we have got to get people to believe that the impossible is possible. We’re trying to cure cancer. One of the things that we’re doing with Genentech is essentially, Mostafa talked a lot about cell therapy, but that is what we’re trying to do. Vein to vein, we’re trying to essentially see how your immune system sees your individualized cancer, take that out, reprogram it, put it back in, 30 days, and have an individual therapy.
So to do that, people have to believe, and that means you have to create an environment, you’ve got to create a set of values and culture that really allows it. People make companies, right?
Ursheet Parikh:
Yep.
Chad Robins:
We’ve got great technology and IT, but it’s the people who make companies. I call myself a CCO or the chief cultural officer, and probably the most important job that I do is to hire and retain the best talent and create a culture that makes people believe that we can do the impossible.
Ursheet Parikh:
That is so well said and it’s something we believe in so deeply. I think we’re almost out of time, and so I was going to leave with the last question for Mostafa, on what would you pick as one silver lining coming onto the other side of COVID?
Mostafa Ronaghi:
I think the opportunity in biology has expanded vastly, and I’m happy to see a lot of entrepreneurs from other spaces actually coming into the biology space, like Chad. It’s amazing to have those mindsets. The funding environment is actually very healthy, and this is going to continue for the next few years. Love to see foundational technologies and also foundational therapies, therapy platforms that will emerge in the next decade.
Ursheet Parikh:
That is awesome. Thank you so much, Chad, Mostafa, Yan, Diego. Delight to have this conversation with you.