Twist’s synthetic DNA is re-inventing the fields of medicine, agriculture, industrial chemicals, and data storage. Ginkgo has developed custom strains of organisms to make fragrances, fertilizers, lab-grown meat, hunt for natural antibiotics, and engineer probiotics. Hear about the founder journeys of these two iconic entrepreneurs from inception through IPO and beyond, and how they are changing our world for the better.
Transcript
Arvind Gupta:
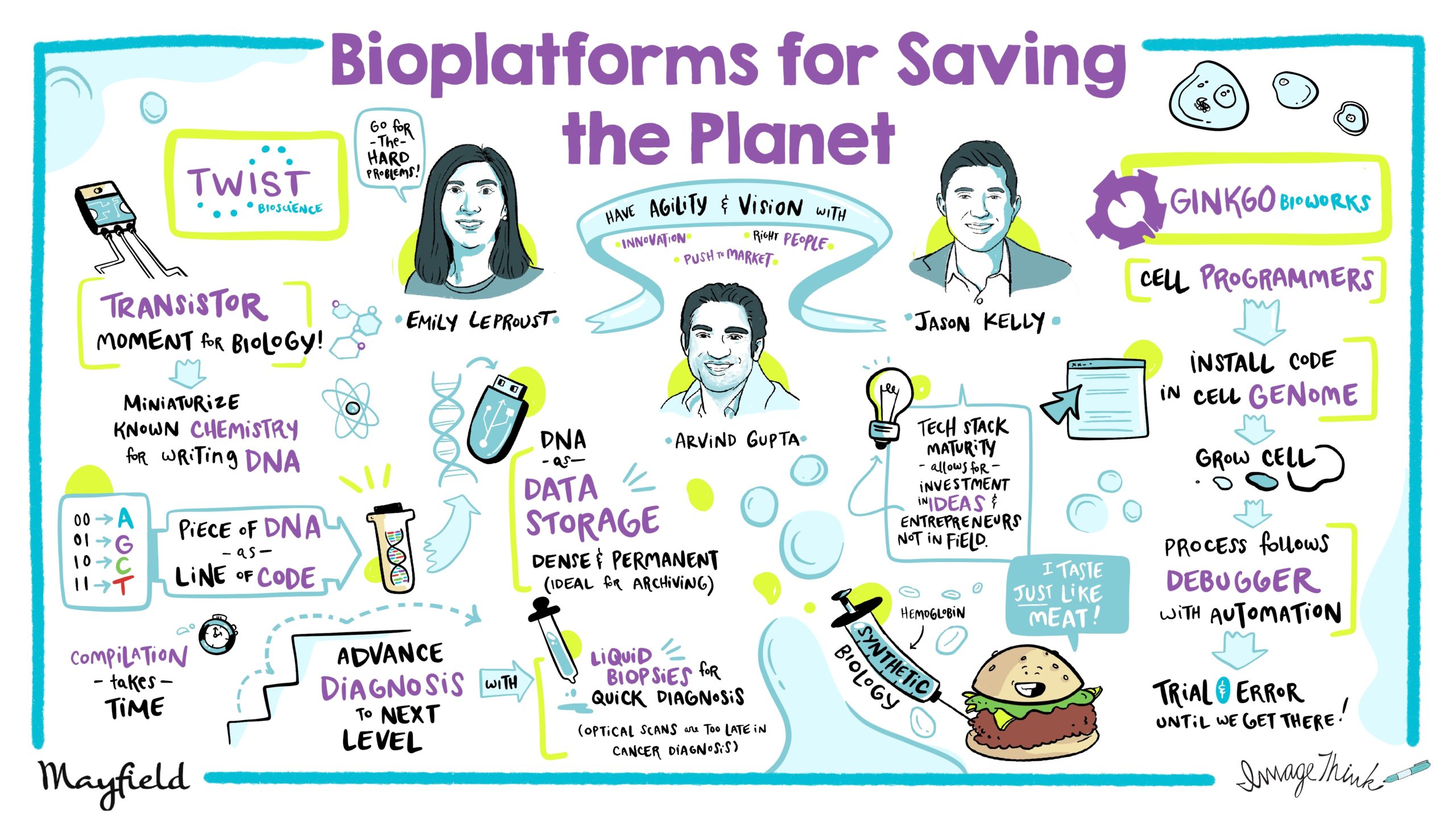
It is my pleasure to introduce two powerhouses of the current synthetic biology revolution, Emily Leproust, CEO of Twist Bioscience, and Jason Kelly, CEO of Ginkgo Bioworks. Twist’s synthetic DNA is reinventing the fields of medicine, agriculture, industrial chemicals, and data storage. Ginkgo has developed custom strains of organisms to make fragrances, fertilizers, alternative proteins, hunt for natural antibiotics and engineer probiotics. Both companies are leaders in the current biology revolution. I’m super excited to hear their unique perspectives from the cutting edge of our future. Emily, this is the transistor moment for biology as a technology. If we’re to continue that computer metaphor for biology, how do you see writing DNA as being able to write the software for life?
Emily Leproust:
Thanks Arvind, thanks for having me and you’re right, that it is the transistor moment – what happened with Fairchild and with the companies in Silicon Valley when they decided to manufacture transistors and miniaturized them. That really unleashed all the amazing things we’ve seen with computers and at Twist that’s what we’re doing. We’re taking the known chemistry for writing DNA, miniaturizing it and we can write DNA better than before. And basically every piece of DNA we make is kind of like a line of code. And so our customers buy a bunch of lines of code and then they have to…
Jason Kelly:
Have to pay by the bin.
Emily Leproust:
Yeah, yeah. To compile them. And so, we send them to Ginkgo and many others and unfortunately for the field, bio is still difficult. And so that means that our customers need a lot of lines of code to combine them and find the one that works for them. And so what will happen is as there are more lines of code compiled, there’ll be a bigger knowledge base. And people will be able to advance biology much faster at the same time as we keep shrinking the DNA and keep blowing the curve, increasing the throughput.
Arvind Gupta:
Fascinating. And so really you’re seeing DNA writing as being, not just putting together base pairs, but actually the compilation layer for the entire language of life.
Emily Leproust:
Yeah, exactly. And I am a very bad coder. I was a bad coder, now I’m a terrible coder. But when I used to code every time I would write a line I would have to compile right away to make sure that the code worked. I’m a chemist by training. So I have some excuses, but yeah, exactly. That computation takes time. Our customers use the design, build, test, learn engineering principle. And so our customers design the line of code. We build it and then they go test it and, and they learn from it.
Arvind Gupta:
Got it. And so you mentioned that you and Ginkgo are working together. So Jason, how is Ginkgo using the software of life to make organisms do useful things for humanity and further to that are organisms actually the programming language of life?
Jason Kelly:
Yeah. So, well, I think I’ll start just to mention, Emily kind of mentions off the cuff that they print DNA, but let’s just pause for a minute of how insane that is. Right. So just for, for folks who might not know this field, right? This literally means going in a computer you’re typing, ATC, GGG up into the kind of thousands of letters of code, you’re hitting print. And then off goes in order to Twist and they literally build the piece of DNA that you want. Right. And then our version of sort of installing code in a cell means that you’re going to open the genome of a cell. So think of like a bacterial cell would have a three million letter piece of DNA that tells that thing how to swim and grow and eat. And you’re going to install maybe 10,000 new letters of code in there.
Right? And you’re going to do that by opening the genome, kind of cut and pasting it in. If you’ve heard of things like CRISPR, that’s the kind of technology that allows that cutting and pasting, you put that new DNA in and then the cell reads it and executes it. Right. And so really that sort of programming metaphor, it’s pretty strong here, right? DNA is literally digital code and Emily has the world’s biggest compiler and you pay by the bit. Okay. Right. That’s what’s going on. And what Ginkgo does is we operate as essentially cell programmers, right? So we’re making use of Emily’s compiler, we’re Twist’s biggest customer ordering that DNA. Right? And then when we get it shipped to us, we install it in the genome, grow the cell up, and then basically run a debugger, right.
I’m sitting in front of a 200,000 square foot debugger, which is basically a bunch of robotics and all automation that opens the cell up, looks at what happened based on the changes you just made to the DNA. Is it making the right proteins, the right small molecules, what’s going on? Because as you know, as a software developer, you’re not going to get it right the first time, you’re going to want to see the output of that debugger. You’re going to want to make some changes to the code, hit compile again, get another order from Emily, put it in and see if it worked and go through that. Ultimately, hundreds of thousands of versions of that code until you get the one that does what a customer wants. And so that’s Ginkgo’s business. We essentially operate as an outsource programming shop. We have our own debugger. We order from Emily’s compiler, we build you a cell, and then you, our customer go off and bring that, that cell app to market. And we take a royalty kind of like an app store economy, basically.
Arvind Gupta:
Wow. And so really, this metaphor’s working out quite well, all the way to the consumer end that you’re talking about Jason.
Jason Kelly:
Yeah. I mean, to give you some examples, we have customers in the animal-free meat space, right. Who are engineering and you know this well Arvind.
Arvind Gupta:
Yes, I’m familiar.
Jason Kelly:
IndieBio really launched this whole area. But, what people are looking for is an animal-free product that still tastes good. And the way you do it is you basically engineer cells to produce animal proteins by taking the code, like a company, like Impossible Foods would take the code for hemoglobin. Right? Which is the protein that makes blood red. They put that ATCGG in a computer, hit print, get the DNA from Emily, install it into a brewer’s yeast, brew it up and instead of beer coming out, Hemoglobin comes out, you add that to a burger and suddenly it smells right and tastes right and cooks right. It’s an Impossible Whopper. That’s a consumer product with synthetic biology on the backside. There’re many things like that that we’re working with. We work in the agricultural space on microbes that produce fertilizers. The applications for this are quite broad.
Arvind Gupta:
Yeah. And I think you’re getting to the actual underlying power of biology as a technology. Right. I mean, how many industries did you name just rattling off your customers?
Jason Kelly:
Well here’s, what’s interesting. Again, people that understand computers get this, right? If you were like, what industries will computers impact? And, the answer is, well, every industry that uses information, right? Because fundamentally a computer is a programmable device that moves bits around, it moves information around. So what did it disrupt? Media, telecom, finance, advertising, anything that involved pushing bits, right. What didn’t computers disrupt, hamburgers. Because hamburgers aren’t made out of bits, right. Hamburgers are made out of atoms. And biology, programmable, I swear to God, it runs on digital code. It’s crazy. Okay. You put new code in, it does new things, but it doesn’t move bits. It moves atoms. And so if you think about the industries that bio and engineered cells are going to disrupt, it’s all the physical goods industries, and yes, that’s going to range from pharmaceuticals to building materials, to agriculture, to medicines, right down the list. Anything with an atom.
Arvind Gupta:
Fantastic way of putting it. Jason, thank you. And for me personally, I’m so excited because I think we could be using this technology to fight climate change and improve human health and all of the above, and really advance that quite quickly. And so Emily, you’re talking about DNA printing, right. And actually making these, but I know that Twist is doing so much more than just that or what else can we do with printing DNA, other than putting base pairs together?
Emily Leproust:
Yeah. So in addition to enabling customers like Ginkgo to do the great thing that Jason just outlined, there’s a few other areas where you can leverage DNA. One of them is in advancing diagnostics to the next level. So if you take the example of cancer, for instance, most cancers are found at phase three or phase four because you feel you have a mass and then they can always be seen in an optical scan, a C scan or an x-ray. And it’s often too late because despite hundreds of thousands dollars maybe even millions of dollars to treat, survival rates are actually pretty low. But you can use the power of DNA to take a blood sample and then use our DNA to extract the cancer genes, and just read that. And so what that means is, and it’s called liquid biopsy and it is delivering the fact that cancer cells are always shedding DNA.
So even though you have just a few cells, here’s a phase one phase two, there is part of that DNA in the blood cell. It’s almost like a shadow of the cancer cell. And then if you can, using Twist we can extract that those cancer mutations and sequence them. And you can get a diagnosis at phase one of phase or phase two, it’s a lot cheaper to treat and the survival rates are much higher. So that new field of liquid biopsy is absolutely enabled by the DNA that we make. Another exciting field, which is very different, is around data storage. So we are frankly, on a path where we are actually running out of sand, running out of silicon. If you plug the amount of data being created and you do the math just in the not so distant future, actually there won’t be enough silicon to store all the data that’s being produced.
There’s a better way to store data than on silicon, that’s using DNA. Our DNA is our hard drive, but you can find men with DNA that’s a million years old and you can see with it. And so DNA has the potential to be basically a per manage storage media. It’s also super dense. So you could put hundreds of Google data centers in a sugar cube, which obviously takes a lot less energy than those millions of square feet data centers. And so the density and the permanence means that we are pushing the technology to enable DNA to be the media of choice for archiving. So it’s not going to be hot data storage where the data is in and out all the time. But if you’re going to read the data once a year or less, DNA is going to be perfect.
That data layer is actually 60% of the market. So those are kind of two exciting new applications of DNA. There are more. We’re mostly working with pharma partners to discover drugs against how to drug target. So we are becoming the drug discoverer of last resort. If you’ve tried to find a drug against a target, you can’t, you come to us and we’ll do it for you. It won’t be cheap. It’ll be a premium offering. You’ll have to pay royalties. But so far, we’re betting a thousand for heart targets.
Arvind Gupta:
That’s so cool, what you guys are doing and storing DNA, storing information in DNA. I think, all of the things you talked about, right? Like this, you said earlier, right. That you have a bigger compiler. Right. So finding drug targets for therapeutics speaks to that database. Right. So the more information you have, the more power you have in biology – is that fair to say?
Emily Leproust:
Yeah. And especially in drug discovery, it’s really a numbers game. People talk of one in a billion, right. You have to try a billion antibodies to find the one. And so if you have the machine that rises to the billion, well, you have a better shot at finding that one antibody. So there’s still a need for traditional ways. The traditional ways where you take a target, like COVID, for instance, and you immunize a rat or a mouse or a rabbit or shark or llama, and then you extract the antibodies produced by the animal. But it doesn’t work for many targets where there is ology between human and the animals. So that’s where we come in and we can really move the needles for those diseases.
Arvind Gupta:
Thank you for that service as well. So, Jason, if we’re going to extend this idea that we’re starting to see emerge here, that there’s a tech stack, for biology. That’s very similar to the tech stack for IT. How do you see the industry expanding? You’re touching all of these different industries through your offering, where do you see this tech stack going and how does it push the boundaries of what’s possible?
Jason Kelly:
I’ll make one quick comment on the data storage thing. I think it’s so cool. DNA is basically the product of who knows how many millions of years of evolution, upstream of DNA to choose that as a medium, to transmit information across generations. You’re going to pass on your genetics to your offspring. DNA is basically what God invented to do that. Right? It makes sense that it’s so information dense. It makes sense that it uses basically no power to store that information. It’s like the evolutionary end to data storage and it’s really cool. So it’s really exciting to connect the dots between that and sort of our electronic data needs.
So I think it’s a super cool area. It’s orthogonal to, well, everything we’re talking about on the cell programming side, right? Like the cell programming side is boy, we need everything for programming cells. We’re so bad at it right now. We’re basically writing assembly code, jamming in bits. We have no higher order to answer your question on the tech stack. There’s no basic. We’re writing assembly. But there’s no reason that stuff can’t get built. There was a day in computing where we wrote assembly. And so that’s kind of what I feel like we’re in today, we’re sort of in the mainframe era of computing, right? Like it’s, you’re still at the metal, you got to be an electrical engineer to be a computer programmer.
That’s sort of the era. And what we’re trying to do with Ginkgo is increasingly extract more and more of that away from the end customer of the platform. In other words, take for example, a company that got built on our platforms, a company called Motif. So it’s in that same animal-free meat space. They want to do egg proteins, milk proteins. They just raised a 226 million dollar round a few months ago. This is a company that didn’t exist three years ago. What they did on day one was they said, all right, we’re not going to build a lab. We’re not going to get a bunch of laboratory equipment, hire a bunch of scientists. We’re going to outsource the cell engineering to Ginkgo’s platform. And they got to make use of all my infrastructure on the first day and really catch up in that area. They didn’t have to be bio technologists.
You know, the folks who run Motif, they’re experts from the food industry. And so I think that’s one of the changes we’re hopeful to make happen as the tech stack matures, is if you’re an entrepreneur and you know some particular area, it could be fashion. It could be building materials, whatever, and biotech could be disruptive in that area. You could use Ginkgo, get the biotech disruption without having to be a PhD scientist. And that allows for a new kind of breed of entrepreneur in the synthetic biology space. I’m really excited to try to make that happen. Cause I think there’s a lot more ideas for what to do with biotechnology and people that can bring those to market than there are PhDs like me in bioengineering. Right. So that’s one of the goals.
Arvind Gupta:
Yeah. No, that’s absolutely right. And it’s really amazing. I know that you guys just signed a partnership with Huue as well, doing sustainable and nontoxic indigo dies. And so yeah.
Jason Kelly:
Genes for jeans.
Arvind Gupta:
That’s right. Watching Ginkgo enable all of these companies I think is just utterly massive.
Jason Kelly:
Again, Huue is going to get access to a few hundred million dollars of infrastructure here and it’s a 15, 20 person company, right? Like that’s the idea, again, this will make total sense to people, in sort of the TechCrunch crowd. Like it’s the same story as AWS, right? Amazon invests billions of dollars in data centers, so you don’t have to. That’s the general model here. It’s not that complicated for the tech crowd – actually, for biotech people, it’s a bit more of a new model. But for the tech and software people, it’s pretty obvious.
Arvind Gupta:
And so Emily, I think Twist is known for its incredible ability to expand and grow. And I think you’re behind so much of that. What’s the driving principle behind your agility and vision? What makes this happen?
Emily Leproust:
Like you said, we are addicted to revenue growth. That’s what we want to deliver. That’s what our investors expect. It’s three things. One thing is innovation. For instance, for data storage, we are increasing the number of features on the silicon ship by a million times, right. We go from a million pieces of DNA to a trillion. We’re skipping the billion. So massive innovation and go for the really hard problems and just crush it. The second is, frankly, very violent commercial execution, right? You just, we have to go take market share. And so we just are very aggressive commercially. And then the third thing is the people. If you start a company, you have A level people, if you’re not careful and you let the A people hire B people, the B people hire C people, and then they C people hire losers and then you’re a big company. So where we’re super careful to only hire A people and enable our employees to do the great things we need.
Arvind Gupta:
Amazing and yeah, clearly, right? You could see immediately why you two are not only super successful leading the way, but also two of my favorite people in the world. So I really appreciate you guys just taking 20 minutes out of your extremely busy days to speak with us and educate us on where the future is going. So thank you guys so much.